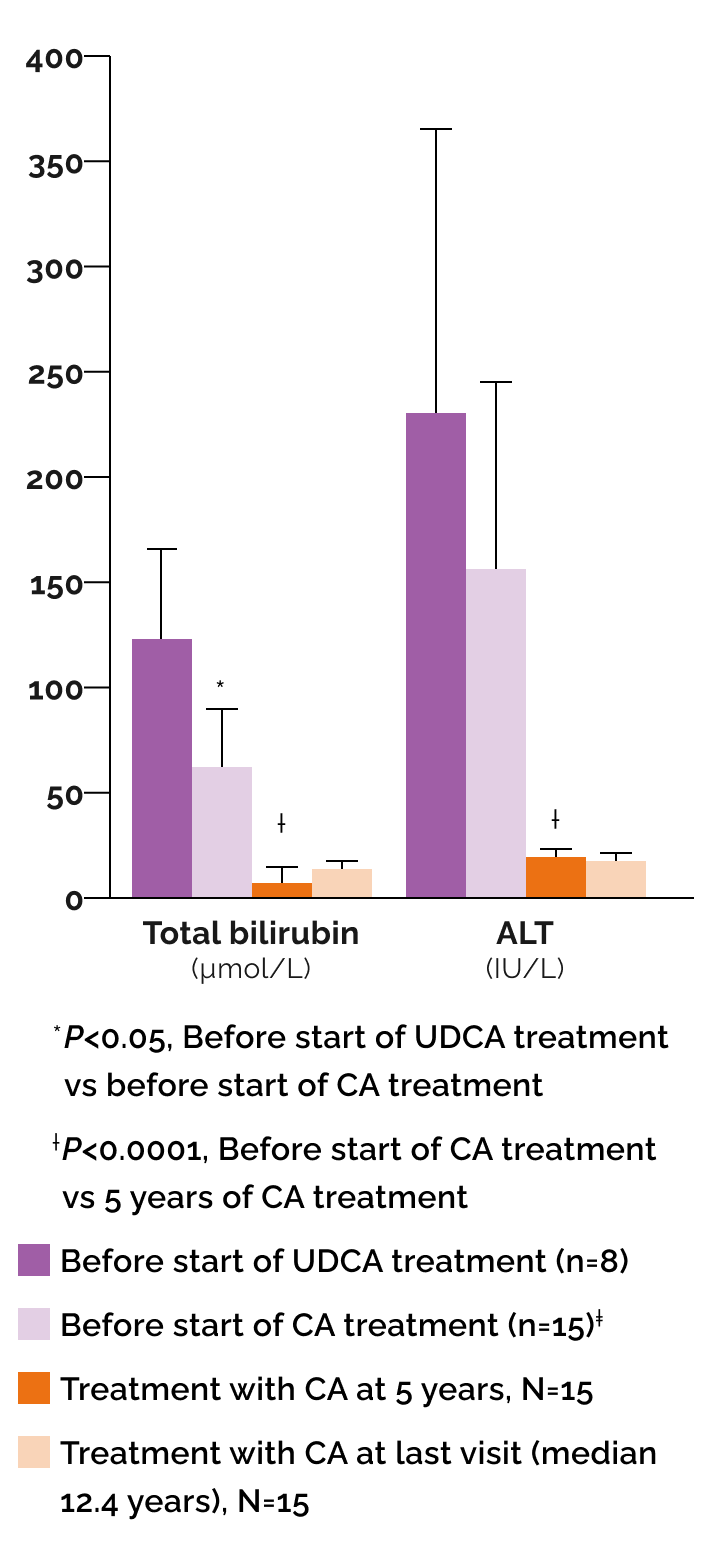
Effect of cholic acid (CA) and ursodeoxycholic acid (UDCA) on biochemical parameters1
‡Includes the 8 patients previously treated with UDCA.
Safety and effectiveness on extrahepatic manifestations, such as neurological symptoms, have not been established. Individual results may vary; not all patients will experience similar levels of response.
In a published case series (N=15) with BASD due to single enzyme defects1:
8 patients received UDCA initially prior to diagnosis of BASD but were switched to CA upon diagnosis
- 7 patients were started immediately on CA
- Median duration of CA treatment was 12.4 years (range 5.6 to 15 years)
After CA treatment, biochemical parameters normalized in all patients and remained normal at follow-up
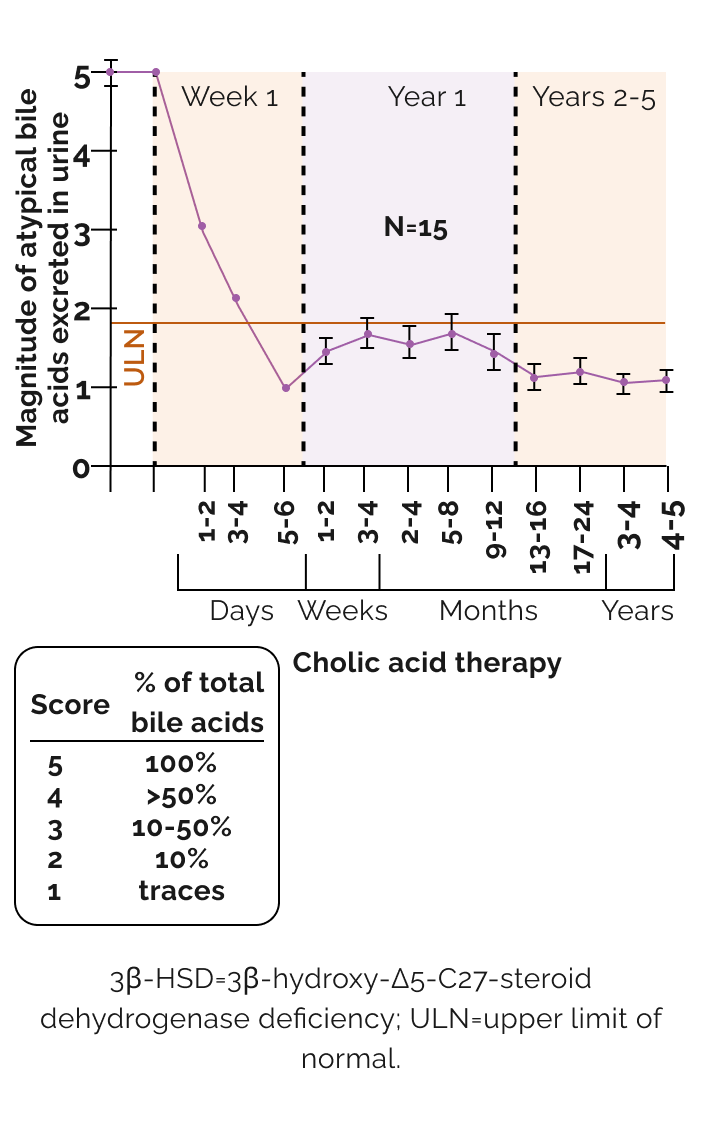
Unlike UDCA, CA is a farnesoid X receptor (FXR) agonist
- Provides physiological feedback
- Suppresses the production of hepatotoxic atypical bile acids
Effect of long-term cholic acid monotherapy on urinary excretion of atypical bile acids with 3β-HSD deficiency2
Years of Clinical Data and Real-World Use
Backed by >18 years of safety data, CHOLBAM has a well-characterized safety profile. There were 12 adverse reactions reported across 9 patients in the clinical trials.1§ In clinical trials, the most common adverse reaction reported was diarrhea (~2%).3
CHOLBAM helps patients absorb what matters. It has been FDA approved since 2015.1
Most common adverse reactions in Trials 1 and 23
Adverse Reactions
Trial 1
Trial 2II
Overall (%)
Diarrhea
1
2II
3 (2%)
Reflux esophagitis
1
0
1 (1%)
Malaise
1
0
1 (1%)
Jaundice
1
0
1 (1%)
Skin lesion
1
0
1 (1%)
Nausea
0
1II
1 (1%)
Abdominal pain
0
1II
1 (1%)
Intestinal polyp
0
1II
1 (1%)
Urinary tract infection
0
1II
1 (1%)
Peripheral neuropathy
0
1
1 (1%)
§Clinical safety experience with CHOLBAM consists of3:
- Trial 1: a non-randomized, open-label, single-arm trial of 50 patients with bile acid synthesis disorders due to single enzyme defects (SEDs) and 29 patients with peroxisomal disorders (PDs), including Zellweger spectrum disorders. Safety data are available over the 18 years of the trial
- Trial 2: an extension trial of 12 new patients (10 SED and 2 PD), along with 31 (21 SED and 10 PD) patients who rolled over from Trial 1
Safety data are available for 3 years and 11 months of treatment. Adverse events were not collected systematically in either of these trials. Most patients received an oral dose of 10 to 15 mg/kg/day of CHOLBAM.
IIAdverse reactions that occurred in new patients.3
Patients taking CHOLBAM should be carefully monitored.
CHOLBAM is simple for patients to start
Help get your patients with BASD started on CHOLBAM
INDICATIONS AND LIMITATIONS OF USE
CHOLBAM® (cholic acid) is a bile acid indicated for
•
Treatment of bile acid synthesis disorders due to single enzyme defects.
•
Adjunctive treatment of peroxisomal disorders, including Zellweger spectrum disorders, in patients who exhibit manifestations of liver disease, steatorrhea, or complications from decreased fat-soluble vitamin absorption.
LIMITATIONS OF USE
The safety and effectiveness of CHOLBAM on extrahepatic manifestations of bile acid synthesis disorders due to single enzyme defects or peroxisomal disorders, including Zellweger spectrum disorders, have not been established.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS – Exacerbation of liver impairment
•
Monitor liver function and discontinue CHOLBAM in patients who develop worsening of liver function while on treatment.
•
Concurrent elevations of serum gamma glutamyltransferase (GGT) and alanine aminotransferase (ALT) may indicate CHOLBAM overdose.
•
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis.
ADVERSE REACTIONS
•
The most common adverse reactions (≥1%) are diarrhea, reflux esophagitis, malaise, jaundice, skin lesion, nausea, abdominal pain, intestinal polyp, urinary tract infection, and peripheral neuropathy.
DRUG INTERACTIONS
•
Inhibitors of Bile Acid Transporters: Avoid concomitant use of inhibitors of the bile salt efflux pump (BSEP) such as cyclosporine. Concomitant medications that inhibit canalicular membrane bile acid transporters such as the BSEP may exacerbate accumulation of conjugated bile salts in the liver and result in clinical symptoms. If concomitant use is deemed necessary, monitoring of serum transaminases and bilirubin is recommended.
•
Bile Acid Binding Resins: Bile acid binding resins such as cholestyramine, colestipol, or colesevelam adsorb and reduce bile acid absorption and may reduce the efficacy of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after a bile acid binding resin.
•
Aluminum-based Antacids: Aluminum-based antacids have been shown to adsorb bile acids in vitro and can reduce the bioavailability of CHOLBAM. Take CHOLBAM at least 1 hour before or 4 to 6 hours (or at as great an interval as possible) after an aluminum-based antacid.
PREGNANCY
No studies in pregnant women or animal reproduction studies have been conducted with CHOLBAM.
LACTATION
Endogenous cholic acid is present in human milk. Clinical lactation studies have not been conducted to assess the presence of CHOLBAM in human milk, the effects of CHOLBAM on the breastfed infant, or the effects of CHOLBAM on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CHOLBAM and any potential adverse effects on the breastfed infant from CHOLBAM or from the underlying maternal condition.
GERIATRIC USE
It is not known if elderly patients respond differently from younger patients.
HEPATIC IMPAIRMENT
•
Discontinue treatment with CHOLBAM if liver function does not improve within 3 months of the start of treatment.
•
Discontinue treatment with CHOLBAM at any time if there are clinical or laboratory indicators of worsening liver function or cholestasis. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
OVERDOSAGE
Concurrent elevations of serum GGT and serum ALT may indicate CHOLBAM overdose. In the event of overdose, the patient should be monitored and treated symptomatically. Continue to monitor laboratory parameters of liver function and consider restarting at a lower dose when the parameters return to baseline.
Please see full Prescribing Information for additional Important Safety Information.
References: 1. Gonzales E, Gerhardt MF, Fabre M, et al. Oral cholic acid for hereditary defects of primary bile acid synthesis: a safe and effective long-term therapy. Gastroenterology. 2009;137(4):1310-1320. doi:10.1053/j.gastro.2009.07.043 2. Setchell KDR. Adolf Windaus Prize Lecture 2004. Defects in bile acid synthesis—specific and treatable causes of metabolic liver disease. In: Paumgartner G et al, eds. Bile Acid Biology and its Therapeutic Implications: Proceedings of the Falk Symposium 141 (XVIII International Bile Acid Meeting). 1st ed. Springer; 2005:3-16. 3. CHOLBAM® (cholic acid) capsules. Prescribing Information. Mirum Pharmaceuticals, Inc.